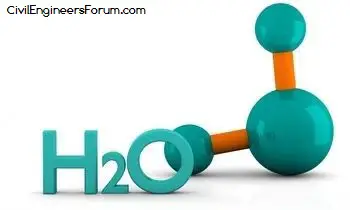Many people do not really take the time to stop and consider the physical and chemical properties of water. Water is essential for life and is the major element of almost every life form. Plants and animals, for the most part, contain more than 60% water. Life would more than likely never have developed on Earth without the clean, goodness of water.
Physical Properties of Water
Water is a very simple atomic structure. It consists of two hydrogen atoms that are bonded to one oxygen atom. The physical properties of water are more significant when you consider the make up of a water molecule.
When a water molecule changes physically, the molecules arrange themselves into very distinctive patterns. The arrangement of the molecules for ice, which is the solid form of water, has a higher volume and decrease in density. This allows the ice to float on top of liquid.
There are also other some physical properties of water.
- There is a high specific heat to water. This is the amount of energy that is required to change the temperature of a substance. It can absorb large amounts of heat energy before it begins to get hot. Water also releases heat energy slowly when it begins to cool. The high specific heat of water allows for the moderation of the Earth’s climate and helps animals and people to regulate their body temperature.
- When water is in a pure state it has a neutral pH. This means that pure water is neither basic nor acidic. The pH of water changes when substances are dissolved into it.
- Heat is conducted more easily through water than any other liquid with the exception of mercury. Large bodies of water, such as lakes and oceans, have basically a uniform vertical temperature profile.
- Water molecules remain liquid between 0 to 100 degrees Celsius. Water molecules remain liquid in most places on Earth because of this large temperature range.
- It is a universal solvent. This simply means that it is able to dissolve a large number of different chemical compounds. Water has the ability to carry solvent nutrients in infiltration, groundwater flow, runoff and living organisms.
- Water has a high surface tension or it is adhesive and elastic. It tends to collect in drops instead of spread out over a surface in a thin film. Water is also able to stick to the sides of vertical structures despite the downward pull of gravity. Plants are able to move water from their roots to their leaves as well as the movement of blood through tiny vessels in the bodies of many animals.
- Water can exist in three physical states of matter: solid, liquid and gas. It is the only substance that can exist in all three states.
- When water freezes, it causes the mass to occupy a larger volume. When water freezes it expands rapidly. Maximum density of water is around 4 degrees Celsius.
These physical properties of water are not the only components that make up water. There are also chemical properties of water to consider which are described below. You can also read my article on water characteristics.
Chemical Properties of Water
Water is a compound comprised of hydrogen and oxygen. It is chemically active and reacts with certain metals and metal oxides. These chemical reactions form bases. When water is introduced to certain oxides of nonmetals, acids are formed. Water reacts with certain organic compounds to form a multitude of products such as alcohols from alkenes.
Chemical properties of water include the fact that water is a polar compound and therefore an excellent solvent. When water is pure, it is a poor conductor of electricity. It is however, a better conductor of electricity than other pure liquids due to its self-ionization.
There are other some chemical properties of water as followings.
- Water’s chemical description is two hydrogen atoms and one oxygen atom. The hydrogen atoms are attached to one side of the oxygen atom giving the water molecule a positive charge on that side and a negative charge on the side without the hydrogen atoms. The structure of water is very basic.
- Water molecules are attracted to each other. This is because opposite electrical charges attract. They tend to clump together making water come together in drops.
- It is the universal solvent since it is able to dissolve numerous substances.
- Pure water has a neutral pH of 7. This means that it is neither basic nor acidic.